Online safety training
Hazardous waste management training
Information for faculty and staff regarding the management of hazardous waste at Earlham.
As a result of the ongoing EPA Region 5 Peer Audit process, teaching and administrative faculty that are directly involved in chemical processes that generate or have the potential to generate hazardous waste should review the information presented here.
Please read the following pages of information regarding hazardous waste management practices. At the end of this presentation, you will be asked to print and sign a sheet recording your comprehension of the information presented.
The Earlham College Chemical Hygiene Plan meets the requirements of the OSHA Laboratory Standard, 29 CFR 1910.1450. Its is available to any laboratory employee or interested party upon request and may be downloaded on the Chemical Hygiene website.
It states that the College is responsible for providing engineering controls (i.e. fume hoods), safety showers/eyewashes, providing personal protective equipment (PPE), and MSDS sheets for chemicals. It also provides basic standard operating procedures (SOP’s) for emergencies and laboratory practice.
Each employee of the College who works with chemicals should know the content of the CHP, know about the availability of MSDS information, and understand the basic SOP’s for chemicals.
If you have suggested updates to the CHP, please inform the Chemical Hygiene Officer.
What does that mean exactly?
Hazardous waste is defined by the EPA by one or more of the following:
- Characteristic Hazardous Waste – has one or more of the following hazardous waste characteristics:
- Ignitability
- Corrosivity
- Reactivity
- Toxicity
- EPA Listed Hazardous Waste – Hazardous waste that is defined in lists published in the Code of Federal Regulations (CFR)
The following pages will explore these categories more in depth.
Related link: EPA Characteristic Wastes
Ignitability
- Liquid : Flash point of less than 140ºF or an aqueous solution containing 25% or more alcohol
- Examples include acetone, methanol, turpentine, mineral spirits
- Solid : Capable causing fire through friction, absorption of moisture or spontaneous chemical changes
- Examples include sodium metal, white/red phosphorus
- Gas : If the compressed gas meets the criteria in 40 CFR 261.21
- Examples include acetylene and hydrogen
- Oxidizer : If the material is an oxidizer (a compound that donates oxygen to a fire and increases its rate), it meets the Ignitability criteria
- Examples include potassium nitrate, ammonium nitrate, potassium permanganate
Corrosivity
- Aqueous liquids that are less than or equal to pH 2.5, or greater than or equal to pH 12.5 and all liquids that fail the Steel Corrosivity test are considered hazardous
- Examples include concentrated acids (such as hydrochloric acid) and bases (such as sodium hydroxide)
Reactivity
- A waste exhibits the characteristic of reactivity if it has any of the following properties:
- Reacts violently with water
- Forms potentially explosive mixtures with water
- Generates toxic gases/fumes/vapors upon contact with water
- Contains cyanide or sulfides that could generate toxic gases when exposed to certain pH conditions
- Capable of detonate if heated under confinement or strongly initiated
- Readily capable of detonation, explosive decomposition or reaction at standard temperature and pressure
Toxicity
- If the waste contains the below listed contaminants at the concentration equal or in excess of the stated regulatory limits:
| EPA Waste # | Contaminant |
CAS #
|
Regulatory Level (mg/L)
|
| D004 | Arsenic |
7440–38–2
|
5
|
| D005 | Barium |
7440–39–3
|
100
|
| D018 | Benzene |
71–43–2
|
0.5
|
| D006 | Cadmium |
7440–43–9
|
1
|
| D019 | Carbon tetrachloride |
56–23–5
|
0.5
|
| D020 | Chlordane |
57–74–9
|
0.03
|
| D021 | Chlorobenzene |
108–90–7
|
100
|
| D022 | Chloroform |
67–66–3
|
6
|
| D007 | Chromium |
7440–47–3
|
5
|
| D023 | o-Cresol |
95–48–7
|
200
|
| D024 | m-Cresol |
108–39–4
|
200
|
| D025 | p-Cresol |
106–44–5
|
200
|
| D026 | Cresol |
200
|
|
| D016 | 2,4-D |
94–75–7
|
10
|
| D027 | 1,4-Dichlorobenzene |
106–46–7
|
7.5
|
| D028 | 1,2-Dichloroethane |
107–06–2
|
0.5
|
| D029 | 1,1-Dichloroethylene |
75–35–4
|
0.7
|
| D030 | 2,4-Dinitrotoluene |
121–14–2
|
0.13
|
| D012 | Endrin |
72–20–8
|
0.02
|
| D031 | Heptachlor (and its epoxide) |
76–44–8
|
0.008
|
| D032 | Hexachlorobenzene |
118–74–1
|
0.13
|
| D033 | Hexachlorobutadiene |
87–68–3
|
0.5
|
| D034 | Hexachloroethane |
67–72–1
|
3
|
| D008 | Lead |
7439–92–1
|
5
|
| D013 | Lindane |
58–89–9
|
0.4
|
| D009 | Mercury |
7439–97–6
|
0.2
|
| D014 | Methoxychlor |
72–43–5
|
10
|
| D035 | Methyl ethyl ketone |
78–93–3
|
200
|
| D036 | Nitrobenzene |
98–95–3
|
2
|
| D037 | Pentrachlorophenol |
87–86–5
|
100
|
| D038 | Pyridine |
110–86–1
|
5
|
| D010 | Selenium |
7782–49–2
|
1
|
| D011 | Silver |
7440–22–4
|
5
|
| D039 | Tetrachloroethylene |
127–18–4
|
0.7
|
| D015 | Toxaphene |
8001–35–2
|
0.5
|
| D040 | Trichloroethylene |
79–01–6
|
0.5
|
| D041 | 2,4,5-Trichlorophenol |
95–95–4
|
400
|
| D042 | 2,4,6-Trichlorophenol |
88–06–2
|
2
|
| D017 | 2,4,5-TP (Silvex) |
93–72–1
|
1
|
| D043 | Vinyl chloride |
75–01–4
|
0.2
|
View the EPA’s Listed Wastes — “F-List,” “K-List,” “P-List” and “U-List”
The EPA also has a number of specific wastes that it has determined as hazardous, these are the listed wastes.
- The F-List : Wastes from non-specific sources. These are from largely from common manufacturing and industrial processes, however, Earlham does occasionally generate F-List waste.
- The K-List : Source specific wastes. These are wastes generated from specific industries and specific industrial processes.
- The P-List and U-List : Discarded chemical products. These are discarded, unused chemicals, such as legacy chemicals that are disposed of as hazardous waste. Earlham generates this type of waste. Special consideration is given to the P-List wastes as additional regulatory scrutiny is afforded these chemicals upon disposal.
Please contact the Chemical Hygiene Officer before discarding P-List or U-List chemicals.
Who makes the Hazardous Waste Determination?
The Chemical Hygiene Officer, in consultation with the faculty or staff member producing the waste, makes the waste determination. This is done to simplify regulatory concerns.
For current & on-going processes:
The Chemical Hygiene Officer is aware of the current, and on-going chemical processes at Earlham that produce hazardous waste and determinations have been made. If you believe there a process that the CHO does not know about, or if a significant change is made that affects the volume or composition of the waste generated, please contact the CHO.
For new chemical processes:
Please contact the Chemical Hygiene Officer in advance of starting your chemical process. This gives the CHO time to research the process and make a hazardous waste determination, if necessary. Once the process has been reviewed by the CHO and any waste determinations made, it is only necessary to contact the CHO when a waste container is full, or if any significant change is made that would affect the volume or composition of waste generated.
The Chemical Hygiene Officer is your resource to ensure environmental compliance at Earlham College.
Containers for Hazardous waste do not need to be anything special. Regulations (and common sense) dictate that they should be compatible with the waste they are to contain (for example: a metal container would not appropriate for acid-containing waste).
Containers must be labeled, at a minimum, with the following items:
- The words “HAZARDOUS WASTE”
- A description of the waste -(Include the waste stream type if possible; common waste streams at Earlham include:
- Non-Halogenated Solvents: acetone, alcohols, mineral spirits
- Halogenated Solvents: methylene chloride, chloroform
- Heavy Metals : arsenic, barium, cadmium, chromium, lead, mercury, selenium, silver)
- Others (corrosive waste, reactive waste, oxidizer waste, etc.)
- The date waste was first added to the container
- The date the container was full (should never exceed one year)
- Other information (optional, but highly recommended)
- Such as what process the waste is generated from
- Volume date (dates and amount added)
Containers should remain closed at all times, except when adding waste to the container.
It is highly recommend that waste containers be placed in some type of secondary containment, such as a plastic bin, in case of a spill or overflow. Also waste containers should be accompanied by some type of means to clean up an accidental spill or overflow (a bucket of cheap kitty litter works well.)
If you need a container, label, or if you have a filled container, please contact the Chemical Hygiene Officer.
Earlham’s normal waste disposal is in May/June of each year. Waste will be picked up at that time unless other arrangements have been made.
The Sanitary Sewer:
What can go down the drain?
During the waste determination process, the CHO will determine whether or not you may put the remains of your chemical process down the sink to the sanitary sewer.
In general, non-water soluble items (such as powders, solvents, paints, paint thinners, etc.) should never be put down any drain. Some of these items may not necessarily be regulated as hazardous waste, but should still be managed in a proper manner.
Examples of acceptable discharges to the sanitary sewer:
-
Non-hazardous, safe, water-soluble solutions
- Examples include solutions of sodium chloride, sucrose, glucose, urea
-
Small amounts of dilute acids or bases
- Can neutralize to appropriate pH with sodium bicarbonate (baking soda)
-
Very small amounts of water soluble solvent resulting from glassware cleaning
- Acetone/Ethanol in amounts less than 5mL
- Collection is recommended if possible
Additionally, the Richmond Sanitary District has approved Earlham’s discharge of currently used fabric dye solutions (Art Dept. – Weaving) and certain currently used biological dye solutions in the Biology and Chemistry departments (in negligible quantities only).
Examples of unacceptable discharges to the sanitary sewer:
-
Non water-soluble solvent (any quantity)
- Including hexanes, paint thinner, turpentine, mineral spirits
-
Oils, greases and paints
-
Pickling solution (any quantity)
-
Strong acids or bases without neutralization
-
Anything substance that meets hazardous waste criteria (from earlier pages)
Used Oil and Aerosol Cans
These are items that could be considered hazardous waste, but the EPA has created a special category for them since they are generated universally by most businesses, factories and colleges.
Items that are considered Universal Waste:
- Spent Batteries
- Including lead acid, lithium, nickel-cadmium, nickel chemistry, silver chemistry
- Used Lamps
- Including fluorescent, sodium, neon, high intensity discharge, metal halide
- Mercury switches from thermostats
The requirements for Universal Wastes are similar to hazardous waste rules:
- UW must be placed in structurally sound and compatible containers
- UW must be labeled with the words “Universal Waste,” a description of the waste, and the date of first collection
- Examples include: “Universal Waste – Batteries – 6/28/11”
- Collection period must never exceed one year
- UW containers must be closed at all times, except when adding waste to the container
- Submit for disposal (to the CHO) before collection has exceeded one year
Please contact the Chemical Hygiene Officer or Maintenance for containers, labels, UW collection, or if you have a container that is full.
Used Oil – Waste oil is generated in many departments on Campus (in vacuum pumps, cutting devices, engines, etc.). The rules for used oil are very similar to UW.
- Collect used oil in structurally sound and compatible containers
- Label, at a minimum, with the words “USED OIL” and the date of generation
- Submit for recycling (to the CHO) before collection has exceeded one year
Aerosol Cans – While these are not Universal waste, they still need to be managed properly. Please contact Earlham Maintenance if you have an aerosol can for disposal. Earlham maintenance has a puncture device that depressurizes the can and absorbs any release from the can making it non-hazardous for recycling.
“Old Stuff I Don’t Want Anymore”
Legacy items are chemicals and waste products from days gone by. Many of these old chemicals result from faculty who retired or left, and failed to dispose of chemicals that would not be reused by the remaining faculty members.
During the 2010 EPA Audit process, numerous legacy items were identified and disposed of in a one time, special disposal at a cost of $14,000.
To prevent buildup of legacy items in the future, departments should review their chemicals on a regular basis (recommended annually). Departments should contact the Chemical Hygiene Officer with a list of items they do not want any longer. The CHO will attempt to redistribute these items to other departments for reuse if possible.
In addition, faculty that retire or otherwise leave the college should review their research/special project chemicals with their department for reuse potential. If the chemicals are unlikely to be reused by that department, the retiring/leaving faculty member should contact the Chemical Hygiene Officer.
Thank you for taking the time out of your busy life to read this information about hazardous waste regulations.
Please take the time to print the following record sheet indicating that you have read and understand the information presented for record purposes.
Thank you!
Faculty Record Sheet (PDF 17KB)
Please return record sheets in Campus mail to Drawer 8.
Student safety training
This online safety course is designed to fulfill minimum safety training requirements for laboratory students, stockroom workers, and laboratory teaching assistants in the Chemistry and Biology Departments at Earlham College.
This is not a replacement for individual and specific safety instruction from the employee’s supervisor or teaching assistant’s laboratory instructor.
This course should be completed and the summary sheet signed and returned to the Chemical Hygiene Officer to be kept on file.
The Earlham College Chemical Hygiene Plan meets the requirements of the OSHA Laboratory Standard, 29 CFR 1910.1450. Its is available to any laboratory employee or interested party upon request and may be downloaded on the Chemical Hygiene website.
It states that the College is responsible for providing engineering controls (i.e. fume hoods), safety showers/eyewash stations, providing personal protective equipment (PPE), and SDS sheets for chemicals. It also provides basic standard operating procedures (SOP’s) for emergencies and laboratory practice.
Each employee of the College who works with chemicals should know the content of the CHP, know about the availability of SDS information, and understand the basic SOP’s for chemicals.
Detailed SOP’s are developed in each individual lab scenario and will be given to you by your instructor or supervisor.
The main goals of laboratory safety is to identify hazards so that risk can be minimized.
Common Hazards in a Chemistry or Biology laboratory or stockroom include:
- Flammable liquids and solids
- Toxic and Carcinogenic Compounds
- Oxidizers
- Reactive Materials
- Peroxide formations
- Gas Cylinders
- Electrical Hazards
- Physical Hazards
In the following pages, all of these types of hazards will be discussed, as well as ways to minimize the risks associated with them.
For chemicals, each departmental area should have a SDS catalog with information on chemicals in that department. SDS information can also be found by searching on the internet.
SDS sheets provide valuable information that will assist you in making decisions on how to best minimize your risk by indicating the hazards of each substance.

The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) is an update to the Hazard Communication Standard (HCS) which now gives workers not only the right to know about hazards in their work place, but the right to understand these hazards.
There are two major updates to the HCS revision of 2012: Safety Data Sheets and hazard classification
Safety Data Sheets
Safety Data Sheets (SDS) were formerly known as Material Safety Data Sheets (MSDS). Under HCS 2012, SDS need to be in a uniform format. The sections for the new format and information these sections contain can be seen below (Source). Each lab should have and SDS binder with SDS for all of the chemicals used in that lab.

Section 1: Identification
Includes product identifier; manufacturer or distributor name, address, phone number; emergency phone number; recommended use; restrictions on use.
Section 2: Hazard(s) identification
Includes all hazards regarding the chemical; required label elements.
Section 3: Composition/information on ingredients
Includes information on chemical ingredients; trade secret claims.
Section 4: First-aid measures
Includes important symptoms/ effects, acute, delayed; required treatment.
Section 5: Fire-fighting measures
Lists suitable extinguishing techniques, equipment; chemical hazards from fire.
Section 6: Accidental release measures
Lists emergency procedures; protective equipment; proper methods of containment and cleanup.
Section 7: Handling and storage
Lists precautions for safe handling and storage, including incompatibilities.
Section 8: Exposure controls/personal protection
Lists OSHA’s Permissible Exposure Limits (PELs); ACGIH Threshold Limit Values (TLVs); and any other exposure limit used or recommended by the chemical manufacturer, importer, or employer preparing the SDS where available as well as appropriate engineering controls; personal protective equipment (PPE).
Section 9: Physical and chemical properties
Lists the chemical’s characteristics.
Section 10: Stability and reactivity
Lists chemical stability and possibility of hazardous reactions.
Section 11: Toxicological information
Includes routes of exposure; related symptoms, acute and chronic effects; numerical measures of toxicity.
Section 12: Ecological information
Section 13: Disposal considerations
Section 14: Transport information
Section 15: Regulatory information
Section 16: Other information
Include the date of preparation or last revision.
Hazard Classification
In the 2012 HCS update, GHS pictograms are used to quickly convey specific information about the hazards of the chemical to the user of the chemical. Below are the nine different HCS Pictograms and hazards associated with those pictograms.
Classifications can be ranked from 1 (most severe) to 4 (least severe) which is opposite to the NFPA 704, as you will see next.
Quick Reference wallet cards with GHS pictograms and SDS sections are available in the Chemical Hygiene Office, Stanley Hall 253.
Related link: View more information on NFPA 704
What do they mean?
The National Fire Protection Association has developed a standard system for identifying chemical hazards. It consists of a diamond with four fields indicating hazards on a 1-4 scale for health, flammability and reactivity and a special field for unique hazards. You may encounter these symbols and numbers in the laboratory environment.
Health – Blue
- 4. Very short exposure could cause death or major residual injury.
- 3. Short exposure could cause serious temporary or moderate residual injury.
- 2. Intense or continued but not chronic exposure could cause temporary incapacitation or possible residual injury.
- 1. Exposure would cause irritation with only minor residual injury).
- 0. Poses no health hazard, no precautions necessary.
Fire – Red
- 4. Will rapidly or completely vaporize at normal atmospheric pressure and temperature, or is readily dispersed in air and will burn readily. Flash point below 23°C (73°F).
- 3. Liquids and solids that can be ignited under almost all ambient temperature conditions. Flash point below 38°C (100°F) but above 23°C (73°F).
- 2. Must be moderately heated or exposed to relatively high ambient temperature before ignition can occur. Flash point between 38°C (100°F) and 93°C (200°F).
- 1. Must be pre-heated before ignition can occur. Flash point over 93°C (200°F).
- 0. Will not burn.
Reactivity – Yellow
- 4. Readily capable of detonation or explosive decomposition at normal temperatures and pressures
- 3. Capable of detonation or explosive decomposition but requires a strong initiating source, must be heated under confinement before initiation, reacts explosively with water, or will detonate if severely shocked
- 2. Undergoes violent chemical change at elevated temperatures and pressures, reacts violently with water, or may form explosive mixtures with water
- 1. Normally stable, but can become unstable at elevated temperatures and pressures
- 0. Normally stable, even under fire exposure conditions, and is not reactive with water
White – Special
- W – reacts with water in an unusual or dangerous manner
- OX or OXY – Oxidizer
- COR– Corrosive; strong acid or base
- ACID and ALK are more specific
- BIO – Biological hazard
- POI – Poisonous
-

– is radioactive
- CRY or CRYO – Cryogenic
*Note: W and OX are the only approved symbols in the NFPA 704 standard, however the above listed additional symbols are sometimes used in an unofficial manner.
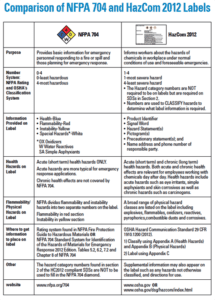
Please see the chart below for a concise comparison between NFPA 704 and HazCom 2012/GHS labels (Source).
Flammable liquids and solids are common in Chemistry and Biology laboratories. Some common flammables include:
- Ethanol
- Isopropanol
- Ether
- Acetone
- Methanol
- Ethyl Acetate
- Hexanes
Flammable materials should be always kept away from open flames (i.e. a Bunsen burner), hot surfaces (i.e. hot plates), electrical equipment (i.e. balances- many laboratory fires have started due to flammable liquid/vapor contact with balances), static electricity and other ignition sources.
Minimize the amount of flammable material out at any one time, especially those with low flash points. The flash point of a substance is the lowest temperature it can form an ignitable mixture in air. Chemicals with flash points below 73ºF are listed as the most dangerous as they are ignitable in air at room temperature. Flashpoints are commonly listed on the chemical label.
Flammable materials should be stored in flammable cabinets, with only small squirt bottles allowed to be stored on bench tops.
When using flammable materials or being present in a laboratory with stored flammable materials, always be aware of the locations of fire extinguishers and fire alarms in case of an emergency.
Related link: Permissible Exposure Limit (PEL) Information
Toxic compounds are instantly a concern in any laboratory working with chemicals.
First, toxicity is relative to the dose and the exposure for each chemical. Any substance, even water, can be toxic, but it is the amount of substance required to cause toxic effects on the body that is important.
LD50 Values
A common feature of MSDS sheets is the LD50 which stands for the dose at which the mortality rate for a given organism is 50%. LD50‘s are listed as mg/kg, which means mg of substance (dose) divided by the mass of the organism in kg. The lower the LD50, the more toxic a substance is.LD50‘s for common substances for rats are listed below:
| Substance | LD50 (mg/kg) |
Substance | LD50 (mg/kg) |
|---|---|---|---|
| Vitamin C (mg/kg) | 11900 | Water | 180000 |
| Ethanol | 10600 | Nicotine | 50 |
| Sodium Chloride | 3000 | DDT | 113 |
It should also be noted that theLD50 can vary based on the route of exposure, with skin exposure usually requiring a higher dose.
LD50‘s however, do not indicate anything about non-lethal toxic effects and therefore should be used carefully. Use in comparing the toxicity of different compounds as well as determining the level of protection to use are some areas the LD50 can assist in.
Permissible Exposure Limits
Permissible Exposure Limits (PEL’s) are regulatory limits from OSHA that limit the amount of a substance in the air in which individuals are working. PEL’s are based on an adult person with an eight hour time weighted average (TWA) but are some times given as a short term exposure limit (STEL) for 15-30 minute working times.
PEL’s often refer to airborne concentrations that may be inhaled and they are typically listed in units of parts per million (ppm).
Working with substances with low PEL’s and those that volatilize easily require the use of engineering controls, such as fume hoods to minimize exposure.
PEL’s in ppm for common substances are listed below:
- Acetone – 750ppm
- Benzene – 10ppm
- Chloroform – 50ppm
- Diethyl Ether – 400ppm
- Formaldehyde – 0.75ppm
- Toluene – 300ppm
Carcinogens
Carcinogenic substances are associated with causing or promoting cancer in humans and animals.
SDS sheets will list if a substance is a carcinogen or suspected carcinogenic agent. In addition, newer containers will indicate such risks.
Common carcinogens include benzene, vinyl chloride, formaldehyde, dioxane, and acrylamide.
Mutagens
Mutagens are substances that change the genetic information of an organism, usually by changing DNA. Mutagens are usually also carcinogens as mutations often cause cancer.
Common mutagens include ethidium bromide, formaldehyde, dioxane, and nicotine.
Teratogens
Teratogens are substances that cause harm to the fetus or embryo during pregnancy, causing birth defects while the mother shows no signs of toxicity.
Common teratogens include ethanol, mercury compounds, lead compounds, phenol, carbon disulfide, toluene and xylene.
It should be noted that carcinogenic, mutagenic and teratogenic effects can and do occur and much lower exposure levels than those required to exhibit toxic effects.
For all toxic, mutagenic, teratogenic, and carcinogenic compounds, the best way to keep safe is to minimize exposure by utilizing engineering controls such as fume hoods, utilizing PPE properly, use and have out only the minimum amount necessary and label the hazards clearly on containers to warn other users to take precautions.
Oxidizing agents or Oxidizers are compounds that generally donate oxygen atoms readily to a reaction, such as a fire. As such, they can increase the rate of reaction of a fire, or even cause explosive detonation given the strength of the oxidizer and the conditions.
Oxidizers are incompatible with organic materials and reducing agents and therefore should be stored accordingly.
Some common oxidizers include:
-
Nitrate compounds (such as Ammonium Nitrate)
-
Chlorate, chlorite, or perchlorate compounds (such as Potassium Chlorate)
-
Oxygen
-
Nitric Acid*
*Special Note– Nitric Acid is generally stored in an acid cabinet, but it should be isolated from organic acids, such as acetic acid, due to risk of fire or explosion.
Special care needs to be taken in labs or storage areas containing special types of reactive materials. These include, but are not limited to, substances that ignite in air, ignite/detonate with moisture and those that ignite/detonate with friction.
A few examples of these substances and their hazards are listed below:
Lithium and Sodium Metals – Reactive in air, ignites in water. Store under mineral oil.
Potassium and Cesium Metals – May ignite or detonate in air or water. Store under mineral oil.
Lithium Aluminum Hydride – May ignite or detonate upon exposure to moisture. May detonate with friction. Keep dry.
White/Yellow Phosphorus – Ignites in air. Store under water. Keep in locked cabinet.
Your supervisor or instructor should notify you of any special reactives in storage or use in your area of employment.
If you are concerned about reactives being stored in your work area, please contact your supervisor or the Chemical Hygiene Officer.
Organic peroxides are highly dangerous compounds that form in some chemicals over time, generally with exposure to oxygen and UV light. These peroxides are highly unstable and can detonate easily, especially upon addition of heat, friction or impact.
The best way to minimize peroxide hazards is to:
- Date all peroxide forming chemicals with date received and date opened
- Dispose of peroxidizable chemicals at expiration date or after 18 months if unopened
A few examples of substances that can form peroxides include diethyl ether, dioxane, and tetrahydrofuran (THF).
Smaller levels of peroxides are typically dissolved in the liquid, but high peroxide levels can lead to solid peroxides precipitating out of solution.
*-* SPECIAL NOTE *-*
If a container of a peroxidizable chemical has solid formation around the lid, DO NOT OPEN IT. The friction of opening the container could potentially cause it to detonate. Arrange for disposal.
If there is significant solid formation inside the bottle, DO NOT MOVE THE BOTTLE. Depending on the substance, the solid could be a highly sensitive organic peroxide that could detonate with a small amount of shock. Arrange for disposal.
Corrosive substances include those with a low (< pH 2.5) and high (pH >12.5) pH value. They can cause acute damage to skin and irreversible damage to eyes.
Common corrosive substances include hydrochloric acid, sulfuric acid, and sodium hydroxide.
When diluting concentrated forms of these substances, always add the corrosive substance (acid or base) to water, not the other way around! This will minimize the danger from splashes.
If you get a corrosive substance on your skin, wash immediately with lots of water. This will minimize the burn. Concentrated base will sometimes not burn right away, but will still be doing damage to your skin!
If you get some in your eyes, flush your eyes in an eyewash for at least 15 minutes, removing any contact lenses. Get medical attention.

Compressed gas cylinders are used in many areas in science and art laboratories and contain a variety of gases in a variety of hazard classes.
Common gas cylinders at Earlham include:
- Oxygen (Oxidizer)
- Propane (Flammable gas)
- Nitrogen (Non-Flammable gas)
- Acetylene (Flammable gas)
- Carbon dioxide (Non-Flammable gas)
- Argon (Non-Flammable gas)
- Helium (Non-Flammable gas)
- Hydrogen (Flammable gas)
To ensure the safety of everyone, gas cylinders should always be secured to a bench or wall. Cylinders with a regulator attached must be secured separately but cylinders with a safety cap may be group chained. Cylinders that are not chained or strapped securely pose a significant hazard to those in the area.
When switching cylinders, leak testing should be performed using soapy water at the connections.
When moving cylinders always have the safety cap attached and use a cart with a chain.
Common sense is a good rule of thumb concerning safety with electrical and physical hazards. Here are a few tips:
Electrical Hazards
Do Not Use equipment that has frayed cords, exposed wires and disabled safety guards or interlocks as these things pose a fire and shock hazard.
Use special caution around high voltage equipment such as electrophoresis apparatus as the higher voltages can deliver a lethal shock. Never disable safety features or use non-standard wiring for these types of equipment.
Do not store or use flammable materials near electrical equipment (including balances) as this can cause a fire. (There have been many fires at colleges and universities involving flammable solvents and balances.)
Physical Hazards
Beware of broken glass, and make sure the proper PPE is used in its cleanup. Make sure broken glass is placed in the proper container and not the regular trash.
Use proper gloves when handling hot or cold items to avoid burns or frostbite.
Make sure isles are clear of chairs, cords, and other trip hazards as these pose a higher danger in the lab where a trip could mean a spill of a dangerous chemical and broken glass.
It is the obligation of Earlham College to provide you with PPE that is suitable to the situation at hand.
Eye Protection
Safety Glasses should used in the laboratory at all times, and in any other area in which chemicals are used. It is recommended that safety glasses also be worn in the stockroom due to the potential of a spill of a bulk material.
Chemical splash goggles and face shields are recommended when using bulk quantities of concentrated acid or base (> 4L). Simple safety glasses may not provide enough protection in the event of a spill of a large amount of caustic material.
Gloves
Use the right type of glove for your situation.
Disposable Latex – General use disposable glove for low amounts of chemical contact. Change gloves often, especially when contaminated.
Disposable Nitrile- General use disposable glove for low amounts of chemical contact. Use for individuals with latex allergy. Change gloves often, especially when contaminated.
Reusable Neoprene – General use reusable glove. Use for acid/base applications and solvents. Wash gloves often.
Kevlar, Heavy Terry Cloth, Zetex, or Asbestos Gloves – Use for high heat applications, such as removing items from an autoclave or oven.
Cryogen Gloves – Use for work with liquid nitrogen, dry ice, or removing items from ultra-low freezers. Do Not Use for work with hot items.
**Note: All gloves have limitations. Chemicals will eventually permeate all gloves, but gloves rated for use with specific chemicals maybe safety used for short time periods.
Lab Coats
Lab coats are recommended for use in lab, especially when working with dangerous and/or corrosive chemicals. Lab coats offer an additional, easily removable layer of protection between potential spills and your body.
Shoes
While not provided by the College, each person in labs or stockrooms is expected to wear proper shoes (i.e. those with a closed toe). Sandals and other types of shoes with holes, vents, etc. on top of the shoe do not offer any protection from a spill of a corrosive chemical.
Other PPE
Other types of PPE, including but not limited to, dust masks, aprons, film badges, air monitoring, etc. will provided if the situation warrants it. Your supervisor or instructor will advise you of such situations.
Using Them to Protect Yourself and Others
Earlham College provides engineering controls in the sciences to help reduce exposure to hazards and keep employees and students safe.
Fume Hoods
Fume hoods work by pulling air from the lab, pulling it into the hood and then out into the atmosphere. They are especially useful when working with dangerous chemicals and those with caustic vapors.
The hood provides two defenses from danger; 1. It pulls dusts, mists, and vapors away from you; 2. The sash, when properly used, provides a physical barrier between you and the hazard, thereby minimizing potential splatter reaching your body.
To properly use a fume hood, follow these guidelines:
- Keep your work at least six inches inside of the hood
- Do not store chemicals in hoods you are working in
- Do not block exhaust areas
- Work with sash at lowest practical level for maximum protection, but always below the safe level (this is just below head level)
- Some hoods may have an ON/OFF switch; leave hoods on at all time
**Note** Laminar flow and bio-safety hoods DO NOT offer protection from chemicals.
Safety Cabinets
Chemicals with special storage considerations maybe stored in safety cabinets. These cabinets are designed to limit potential exposure to chemicals in storage, isolate them from incompatible materials, and to limit fire potential (flammables). Some common cabinets include flammable cabinets (yellow or red), corrosives cabinets (blue), and poison/toxic cabinets.
Other Engineering Controls
Other engineering controls such as glove boxes, isolation rooms, etc. may be provided for use. Your supervisor or instructor will indicate the standard operating procedures for those controls as needed.
Use If Needed
Earlham College provides safety and emergency equipment to all areas using potentially hazardous chemicals.
Safety Showers & Eyewash Stations
Safety showers and eyewash stations are located in and near lab areas that use potentially hazardous chemicals. Safety Showers and Eyewash stations are located in the Chemistry, Biology and Art labs at Earlham College. Know where your closest station is when working with chemicals.
Eye-washes should be used if a chemical gets in the eye. Contact lenses should be removed after a short initial flush of water to aid in the flushing process.
Safety showers should be used if a large quantity of a chemical (especially strong acid or base) is spilled on ones clothing. Affected clothing should be removed to assist in flushing chemicals away from skin. Showers can also be used to extinguish a fire on a persons clothing.
Fire Extinguishers
Fire extinguishers are located in every laboratory and stockroom. The types found in labs include:
Dry chemical type (Ammonium phosphate : ABC Rated; combustibles, liquids, and electrical fires)
Carbon Dioxide (CO2; BC Rated; liquids and electrical fires)
Also, the Chemistry stockroom has a Class D fire extinguisher for combustible metals. It uses sodium chloride to put out metal fires such as sodium, potassium metals, and other metal powders.
Know where the closest appropriate fire extinguisher is when working with chemicals.
Fire Blankets
Fire blankets are available in some laboratories in Biology and Chemistry. Once designed to be used to smother flames on a persons clothes, these wool blankets are no longer recommended to be used for this purpose. Instead safety showers should be used to put out flames, if possible, and then the blanket used to keep the victim warm to prevent shock until help arrives.
Preventing Accidents
Proper lab practice will help keep you and the people around you safe. Here are a few topics that are of great importance:
Labeling – Unlabeled Today, Unknown Tomorrow
When transferring to smaller containers or when making solutions of chemicals, it is very important to label the new container’s contents.
A good label should have the full chemical name (i.e. lead nitrate), concentration (i.e. 1M), date of transfer/dilution (i.e. 7-18-11), any special hazards (i.e. carcinogen) and your initials (i.e. BDE). Using labels with NFPA 704 diamonds is a plus.
Working in the Lab – Always have a partner
It is never a good idea to work alone in the lab. Having a partner greatly increases the chances of you getting timely help if you become incapacitated.
Housekeeping – A Clean Lab is a Safe Lab
Keeping your work area clean is important to your safety and the safety of others. A cluttered workspace can lead to spills, broken glass, and even fires. It is also important to keep chairs, book bags, and other items out of the way to prevent trips and falls.
No Food or Drinks in Labs or Stockrooms
Food and drink in a laboratory is bad practice and an accident waiting to happen. Food and drink in the lab may be subject to contamination with dangerous chemicals, that you could then ingest.
As ridiculous as it may sound, there have been instances of ethylene glycol poisonings resulting from a person accidentally drinking out of the wrong container. Ethylene glycol (commonly known as antifreeze) is a clear, sweet intoxicating liquid that is quite toxic. Its LD50 is only 1.4mL/kg, meaning a 70kg person would require less than 100mL for lethal toxicity, with the LDLO, or the lowest lethal dose for adults being only 30mL or about two tablespoons.
All laboratories generate waste – lots of it. Here are a few guidelines to follow.
Needles & Sharps
Used needles and sharps (razor blades, etc.) should be placed in red plastic sharps containers. If the sharps biohazard contaminated, the sharps container should be marked appropriately and autoclaved when full. If the sharps have been in contact with human biological fluids, the sharps container must be labeled accordingly.
Broken Glass
Broken glass is an inevitability in any lab. Please clean up all broken glass pieces and put in a labeled broken glass box. Do not use these boxes for normal trash or for sharps. If broken glass is biohazard contaminated, then place in a biohazard red sharps container, and autoclave when full.
Chemical Waste
Chemical waste is highly regulated and must be disposed of properly. In general, many water soluble, non-toxic, substances without heavy metals may be disposed of down the sanitary sewer. For organic substances, generally they are segregated as halogenated waste (solvents with halogens, such as chloroform, dichloromethane, etc.) and non-halogenated waste (solvents such as acetone, ethyl acetate, toluene, etc.). Your supervisor or instructor will specify instructions how to dispose of waste properly. If you have any questions or concerns, please contact the Chemical Hygiene Officer.
Regular Trash
You may dispose of solid non-hazardous waste in the regular trash. Examples include used gloves, paper towels, non-contaminated plasticware, etc. Items contaminated with biohazard should be decontaminated before disposal in regular trash. This may mean placement in a biohazard bag and autoclaving the contents before dumpster disposal.
Items contaminated with highly toxic or dangerous materials (such as mercury) should be segregated in a labeled container and disposed of with hazardous solid waste.
**Remember**: The Chemical Hygiene Officer is your resource for information on the proper disposal procedures for your situation.
Keeping Bad Things Out of the Hands of Bad People
Lab security is everyone’s responsibility. Here are a few things to do to keep your lab secure.
- Be Observant!
- Know who is in your lab and who should and shouldn’t be there
- Know what chemicals are brought into your lab
- Know what chemicals are removed
- Control access to storage and research areas
- Keep doors to labs locked when they are not occupied
- Keep doors to storage areas locked when not occupied
- Keep additional locks on storage cabinets containing substances with unique or especially dangerous hazards
- Keep locks on theft prone equipment such as balances
Report to your supervisor if something seems out of place or missing and when was the first time you noticed it that way. If something appears to be stolen, report it to Earlham Security (X1400) as soon as possible.
Earlham College has had thefts of chemicals and equipment in the past. Don’t think it can’t happen, because it has. Vigilance is our best defense.
Safety Training Acknowledgment Form
Thank you for completing the Earlham College Laboratory Safety Training Online Course.
For our records, please download the form below, fill it out, sign it, have your supervisor sign it, and return it to the Chemical Hygiene Officer.
This form is the official record indicating that you have read and understood all of the information presented.
If you have any questions, please contact the Chemical Hygiene Officer before signing the acknowledgement form.
In the event of emergency, call 911 and then notify Public Safety.